Research Article: 2017 Vol: 20 Issue: 2
The MedTech Innovation Course: Description and Initial Experiences with a Novel Collaborative Course Model
Jawad T Ali,University of California
Sarah Mayes,Alafair Biosciences
Heather Haeberle, University of Texas at Austin
Margo Cousins,University of Texas at AustinAbstract
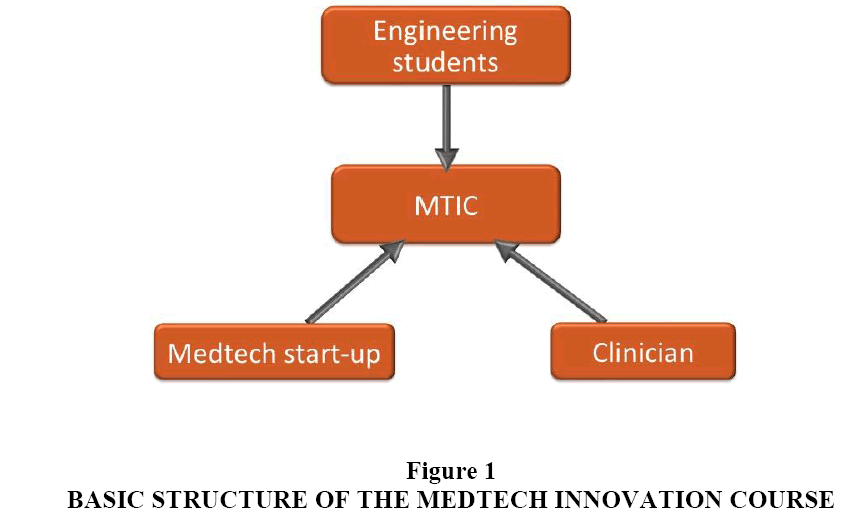
The MedTech Innovation Course (MTIC) seeks to recreate the synergy of the Biodesign Fellowships in an accessible and reproducible format. The aligning needs of engineering students, clinicians, and medtech companies create a fertile environment.
A clinician and biotech company co-sponsors a student engineering team in a structured format. Two trial Courses were conducted at the University of Texas at Austin. The cosponsorship structure was laid out in the initial Statement of Work and intellectual property (IP) agreements were signed before the Course commenced.
The initial Course partnered with an inventor-led start-up with a hydrogel membrane that needed development of a laparoscopic tool to aid in delivery during surgery in order to decrease the formation of harmful adhesions. The second Course utilized lessons from the first and demonstrated the versatility of the model as the team worked with a mobile health platform to develop a careplan for patients with gastroesophageal reflux disease (GERD). Novel survey instruments, a functional careplan, and a clinical trial were successfully designed.
The MTIC addresses the mutual needs of engineering students, medtech companies, and clinicians. Our initial trials prove its feasibility and open the door for future growth.
Keywords
Bio design, Engineering Education, Medical Innovation, Senior Design, Collaboration.
Introduction
Three key needs intersect to create a fertile environment for a new kind of design course, one that brings together clinicians, engineering students and MedTech start-up companies. First, universities teaching engineering capstone design courses strive to offer an authentic design experience that includes multidisciplinary collaboration, a real world problem and mentorship. Prior studies have shown multidisciplinary courses to be superior in terms of job placement and an unmet desire among engineering students for exposure to entrepreneurism (Brooks et al., 2007, Hotaling et al., 2012). Second, clinicians are increasingly looking to participate in activities outside of direct patient care that include connections to industry and academia (Goldberg, 2007). Third, there is a rising tide of MedTech start-up companies that critically need assistance with their design problems including access to resources such as prototyping equipment and insights from medical experts on a modest budget. The MedTech Innovation Course (MTIC) described in this paper attempts to address the aforementioned needs in an accessible, structured and reproducible manner. In essence, a clinician partners with a start-up biotech business to work on a real-world design problem with the capstone project students (Figure 1).
The authors describe our experience with two cycles of the MTIC at The University of Texas at Austin Department of Biomedical Engineering (UT BME) senior design program. A description of each year is provided along with a discussion of lessons learned and steps for future improvement.
Course Design
The MTIC was structured in these initial trials as a partnership with UT BME as one of the many senior design project options for the undergraduate biomedical engineering students to choose from. Each team of students is constructed using the CATME Team Maker (http://info.catme.org). The student teams rank each project and the instructors match teams to a project within their top five choices. UT BME has a $4500 subvention fee that is paid by the sponsoring organizations and helps fund the prototyping resources provided to the students. The students assign their intellectual property (IP) on the project to the sponsor by way of a signed IP assignment agreement (students who do not want to assign their IP are given the option to work on a non-sponsored project by the instructor of the course).
The timeframe of the design course is determined by The University and conforms to the academic calendar, spanning the 8 months from October to May. An example timeline for the UT BME program is given below.
UT BME Senior Design Course Timeline
September 30: Deadline to submit Statements of Work.
October 17: Teams choose from the list of projects and start working with sponsors on the project. Nondisclosure agreements are signed and the subvention fee is invoiced.
December 5: Teams submit a written proposal of their design solution to sponsors and receive feedback over the winter break between semesters.
January 17: Teams return to campus to begin prototyping and testing. Regular progress reports are provided to sponsors and periodic design reviews will be given to the course instructors through the spring semester during this process.
April 24-27: Teams give a final, formal presentation of their finished design, prototype and testing results to sponsors and the instructors.
May 12: Students present final written design report, design notebooks, the prototype and any usable project materials to their sponsoring organization.
An example of how the MTIC schedule fits within the academic year is shown in Table 1. The authors will describe the modifications made for each course to give an idea of possible permutations.
| Table 1 Sample Base Mtic Curriculum ? | |||||||
| Months | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Clinician | Educate on disease, demonstrate end user environment | Clinical feedback on ideas | Input from other clinicians | Clinical testing resources | Provide input on iterative improvements | Final design development/testing | Invite other clinicians |
| Company | Introduce technology, functionality | Guidance based on product needs | Input from outside consultants | Guide detailed design features | Invite start-up/industry team | ||
| Students | Learn about disease process, needs | Brainstorm ideas | Refine design options | Finalize design/initial prototypes | Refine prototype | Present final design solution | |
Initial Experiences
The authors describe the first two iterations of the MTIC detailing distinguishing aspects and lessons learned. The clinician and university stayed the same for both courses while the industry co-sponsor and student team differed. They were both conducted at UT BME and the sponsoring clinician was Dr. Jawad Ali while he was completing his general surgery residency.
The first MTIC partnership was conducted in the 2012-2013 academic year and co-sponsored with Alafair Biosciences, Inc. (Alafair). Alafair is a start-up company based on a proprietary hydrogel film technology. Among other purposes, the film has been proven to reduce harmful intra-abdominal adhesions after surgery that can lead to bowel obstruction (Mayes). Alafair needed to develop an instrument for laparoscopic delivery of a liquid tissue anchor to secure the film after it had been deployed in the abdominal cavity. The modified curriculum is shown in Table 2.
| Table 2 Mtic Modified Curriculum for Laparoscopic Delivery Tool |
||||||
| Months | 1 | 2 | 3 | 4 | 5 | 6 |
| Clinician | Introduce minimally invasive techniques/tools | Determine design constraints such as sterility, handling | Guide material selection, prototyping | Bring in experts, visit advanced prototyping facilities, refine prototype | Weekly meetings, testing. | Use device in animal model |
| Industry | Introduce technology/device needs | |||||
| Students | Learn about rapid manufacturing apparatus | Brainstorming | Prototyping | ?Works like? prototype | ?Looks like? prototype | Final presentation |
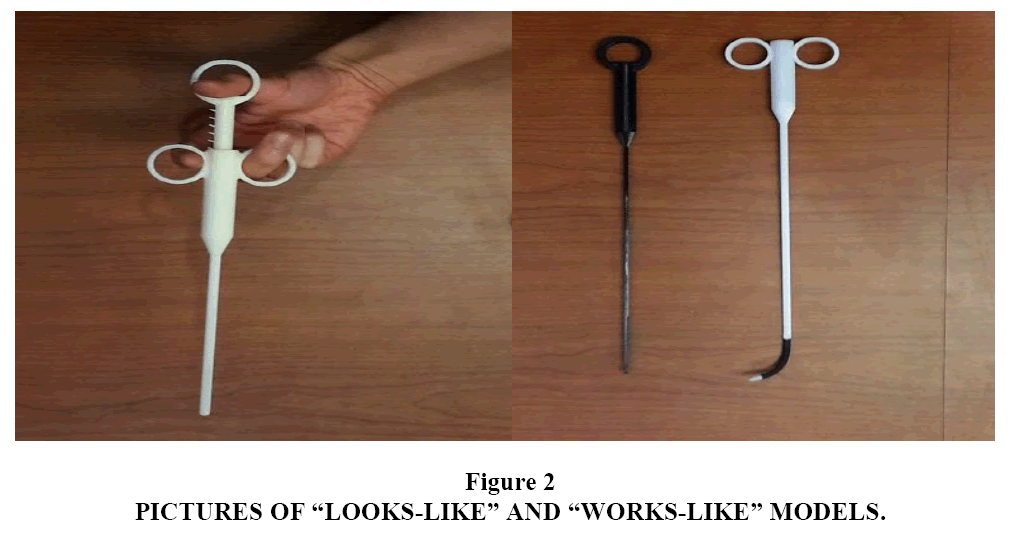
The initial month was used to familiarize team members with the varied aspects of the project. Alafair described the hydrogel technology, current animal testing results, the company?s plan to further develop the laparoscopic applicability of their product and gave design constraints. The clinician provided background on the problem of adhesions after surgery. He also described laparoscopic principles and the operating room environment. The UT BME student team did background research and then went on to brainstorm designs. The second semester began with prototyping and quickly moved to iterative testing and modification. Experts in medical devices, laparoscopic surgery and prototyping provided feedback at key points. The multidisciplinary nature of the team provided a wide range of colleagues and contacts. An important concept was to make two prototypes: a ?looks-like? model to demonstrate the final design?s appearance and a ?works-like? model to demonstrate functionality. They are both shown in Figure 2. The final model was used for laparoscopic testing in a novel hybrid rat model that assessed the efficacy of the biofilm. The design and outcome of the first MTIC model was presented at the 2015 Biomedical Engineering Society Annual Meeting in Tampa, Florida.
The second MTIC partnership was conducted in the 2015-2016 academic year. It was co-sponsored with Filament Labs, Inc., an Austin-based company centered on a mobile care plan delivery application called Patient IO. The modified curriculum for this version of the course is outlined in Table 3.
| Table 3 Mtic Modified Curriculum for Mobile Care Plan Delivery Application | |||||||
| Months | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Clinician | Educate on disease, demonstrate clinic and surgery | Advice on clinical guidelines | Get input from outside experts | Work on IRB/data sharing agreements | Weekly meetings, continue work on clinical trial | Work on study implementation, focus group on care plans | Invite other clinicians |
| Company | Introduce technology, functionality | Feedback on platform aspects | Meet with programmers | Invite Patient IO team | |||
| Students | Learn about disease process and app | Brainstorm care plan goals | Initial care plan design | Iterative care plan modification | Design survey instrument | Simulated trial | Present care plan, study design |
The aims of the second MTIC cycle were very different. Patient IO allows providers to connect with patients and provides them with personalized care plans that can be delivered via a smart-phone application. The objective was to develop a care plan for patients with gastro esophageal reflux disease (GERD) as well as design a clinical trial to assess improvement in patient satisfaction as well as reflux-related quality of life. The team partnered with Dr. Paul (?Tripp?) Buckley, Director at the Baylor Scott & White Heartburn and Acid Reflux Center that includes a comprehensive, multidisciplinary reflux clinic and offers both medical and surgical treatment options.
The initial two weeks were again used to familiarize the UT BME student team with the disease process and design constraints for the care plan. Patient IO representatives discussed the application platform and functionality. Dr. Buckley introduced us to his clinic and allowed the team to observe anti-reflux operations. He also discussed goals for a possible care plan and how it might integrate into clinical practice.
The team then brainstormed care plan possibilities and how they would fit in to different points along the treatment pathway. Initial care plans were discussed with team members as well as other reflux center staff to create care plan ?prototypes.? The authors met with Patient IO programmers to expand on possibilities and were able to introduce new functionality prospects. While the UT BME team worked on iterative improvements in the care plan design, Dr. Ali and Filament Labs continued moving forward with IRB approval and patient data sharing agreements.
After analyzing existing validated survey instruments, the UT BME students consulted with psychometric faculty, reflux center staff and Filament Labs to create a health care application survey that included patient satisfaction with care, mobile application usability and quality of life elements. They also described a method to test its validity.
The final deliverable for the UT BME team was a computational model of a simulated clinical trial, which was developed in consultation with UT statistics faculty. They gave a final presentation and written report to the MTIC partners and to the instructors of the UT BME senior design course. The second course was presented at a platform session of the 2016 Biomedical Engineering Society Annual Meeting in Minneapolis, Minnesota.
Discussion
The MedTech Innovation Course developed as an organic solution to the aligning needs of clinicians, MedTech startup companies and undergraduate engineering students in Austin, Texas. From discussions with biomedical engineering educators, entrepreneurs and physicians it is clear that our situation is representative of many other cities around the country. The authors hope our experience can be valuable and help others conduct similar implementations. The course will be discussed from the perspective of each party then analyze lessons learned and opportunities for growth.
Clinicians are seeking roles outside of traditional patient care, specifically in innovative entrepreneurial pursuits (Majmudar & Harrington, 2015). Reasons for this include labor-intensive documentation requirements that are constantly evolving, increasing bureaucracy and decreasing reimbursement (Goldberg, 2007). While typical bio design fellowships offer a structured opportunity, they generally require relocating and relinquishing fulltime clinical practice for a period of time. During residency this is often not possible and afterwards it is rarely practical. Also, most programs typically involve needs identification and development of novel ideas but not the continuing development of existing ideas or even work in a specific area of interest. Medical schools are often including medical innovation and quality improvement as part of the curriculum (DePasse & Caldwell, 2016). This will further increase the demand for cross-disciplinary collaboration and also improve the ability of physicians to thrive in that role.
At the same time, healthcare start-ups are booming. There are many opportunities for technology to improve, even disrupt, healthcare as the result of several trends. One area in particular is caring for the aging population and their increasing health needs. Another is the epidemic of obesity and its related comorbidities. Also, healthcare reform and regulatory changes open the door for new opportunities (DePasse & Caldwell, 2016). In addition, wearable technology and mobile health applications allow real time collection of large amounts of data and increased engagement, both of which have the potential to revolutionize care. Alafair, for example, benefited from increased interest in venture funding which allowed them to conduct animal trials and further develop their technology. Filament Labs had also earned capital to develop their mobile platform and contracted with a few relatively small clinical enterprises; to expand further they needed to grow their care plan offerings with detailed clinical assessments and include testing of the application?s efficacy. Clinics and hospital systems are difficult to access for small businesses. Conducting clinical studies is notoriously difficult due to IRB approval requirements, patient data access and recruiting a clinical partner within the current healthcare system.
Leaders in engineering education long ago identified a need to provide not just scientific and technical knowledge, but to equip new graduates with the ability to navigate the complex multidisciplinary landscape of today?s medical industry (Wulf & George, 2002). There is a large demand for entrepreneurship education at the undergraduate level as reinforced by a recent Kauffman Foundation report with over 400,000 students attending entrepreneurship related courses annually (Brooks et al., 2007). Working alongside startup companies helps address the lack of entrepreneurship education that up to two-thirds of undergraduate engineering students profess (Duval-Couetil, Reed-Rhoads & Haghighi, 2012). From speaking to numerous biomedical engineering instructors, it is a real challenge to provide undergraduates with design challenges that train them for the dynamics of the real world. Over 80% of these teams do not include disciplines outside of engineering and it can be a struggle to incorporate other professionals (Zuger, 2004). Trying new approaches is necessary to provide a quality design experience for students in an environment of decreasing funding, larger student bodies and multiple demands on both faculty and industry personnel. In addition, graduates have to navigate a complex landscape of tech start-ups and established corporations when looking for jobs. Analysis of a multidisciplinary capstone design course at Georgia Institute of Technology found participants having improved job placement and industry evaluations compared to students in monodisciplinary courses (Hotaling et al., 2012). With over 100,000 undergraduate engineering seniors annually in the United States alone, the need for such courses is vast (Yoder, 2015). Also, universities are taking advantage of technology developed at campuses to bring a return on investment through various forms of technology transfer (Huggett, 2014). Many students are deciding whether a career in medicine or graduate studies is right for them. The MTIC addresses all of these needs and more. There is a focus on mentoring so students can get insight into the start-up and clinical medicine worlds as well as connections to help them succeed. For example, students planning on going into industry were introduced to other start-up companies as well as representatives from larger corporations such as Medtronic. Others that were applying to medical school were connected with clinicians to work with and advice on applying and interviewing.
The decision to co-sponsor a senior design project was daunting due to time constraints on the part of the clinician and business as well as uncertainty about the usability of the final deliverables and the mechanics of co-sponsorship. UT BME was supportive of our involvement and welcomed a partnership MTIC. Other BME programs have expressed interest in such a course as well as difficulty in getting ideal design projects. This openness from the department was critical in getting our participation and achieving success. The subvention fee of $4500, which is charged to each sponsor and helps the teams with costs of materials and prototyping, was waived for the second trial showing their interest after the success of the first trial. Additionally, students working on projects with external sponsors assign all intellectual property rights to the sponsors. This is very attractive and can be a necessity when working with start-up companies developing novel technologies. Future trials may include individualized IP agreements between the MedTech Company, clinicians, students and The University.
When first initiating this course, it may be best for the two co-sponsors to have worked with each other in the past so they have a known dynamic to as a foundation for the co-sponsorship as it will require them to take on roles and responsibilities they may not have much experience with. In particular, clinicians usually don?t have experience in managing technical projects so this may be a key role for the industry member. Such logistics should be discussed beforehand to facilitate scheduling meetings and assigning tasks. If this is not clear among the co-sponsors, it can be confusing for the student team and lead to delays that are devastating to a project on a short timeline. For the first cycle, there was a significant learning curve and delays in progressing from prototyping to testing set back refining the final product. For the second cycle, the clinician assumed the project manager role and had an open discussion at the outset about expected deliverables as well as creating backup plans for when/if unexpected setbacks forced delays. This allowed the team to pivot to a virtual study and work on developing a novel survey when delays in IRB approval and data sharing agreements pushed the actual clinical trial out of the time window. This also highlights the importance of addressing any IRB approval requirements and approvals for patient data access far ahead of time is ideal. Having a hard deadline for initial design and prototyping to be done before the end of the first semester will allow for iterative improvements and final design testing in the second semester. The winter break is a time for the clinician and start-up to work on portions that don?t need heavy student involvement. The final presentation is a good opportunity to invite other interested clinicians and members of the biotech industry. It provides a wider audience for feedback, helps develop interest for future projects and allows for networking among all members including the students. University affiliation gives access to a wide range of experts. The team consulted professors in statistics, chemistry and survey design who were all enthusiastic about offering their services. There was a similar response from other healthcare professionals and engineers from large biotech companies. The expanded network and the ease of collaboration are massive advantages to being part of an exciting course structure with multiple affiliations.
Our experience from the first two trials of the MTIC is encouraging and there are several aspects for future development. A major goal is for it to be an official course offering. This would provide an increased level of legitimacy to improve participation and give leeway for modifications. For example, the opportunity for sponsors to select particular team members among a pool of applicants or for potential co-sponsors to apply and meet each other in a ?speed-dating? type format. In addition, there could be separate instruction for the clinical and business partners by university professors from not only the engineering school but also business, design and medicine. Another aspect to develop is a means for participants to stay connected after the course, effectively creating a network that could collaborate independently of the MTIC. Such networks can be invaluable and an incentive to participate in and of themselves. In addition, the course could be offered between professional schools; for example having a team made up of students from the engineering, medical and business schools or any combination such as engineering and medical students partnering with an industry sponsor. We have discussed the potential for such courses at UT Austin with the existence of excellent engineering and business schools and the recent addition of the medical School. MedTech companies that want to pursue their project further can have the opportunity to continue working with the graduating students in the form of a post-baccalaureate program with a funded stipend. Speaking to many students, this is an exciting opportunity to get start-up experience in the relative security of their home institution and location. It also allows businesses to work with people they know and the university to strategically place resources in projects that have shown success.
The MedTech Innovation Course brings start-up companies, clinicians and engineering design students together in a structured and accessible model. After two trials of the MTIC, we are confident that it is feasible and has tremendous synergistic potential. Each iteration of this course provides additional insights into interdisciplinary team dynamics, possible barriers that must be overcome and the design process in general. We hope this description and analysis is helpful to others attempting similar enterprises and welcome the opportunity for collaboration.
Acknowledgement
We would like to acknowledge the following people for their assistance in writing this manuscript:
1. Lyn Denend, MBA
Director, Academic Programs
Stanford Bio design
2. Albert Huang, MD
Founder/CEO
Allotrope Medical Inc.
References
- Brooks, R., Scott Green, W. & Hubbard, G. (2007). Entrelireneurshili in American higher education. A Reliort from the Kauffman lianel, 1, 1-23.
- Hotaling, N., Burks Fasse, B., Bost, L., Herman, C. & Forest, C. (2012). A quantitative analysis of the effects of a multidiscililinary engineering calistone design course. Journal of Engineering Education, 101(4), 630-656.
- Deliasse, J.W., Caldwell, A., Santorino, D., Bailey, E., Gudaliakkam, S., Bangsberg, D. & Olson, K.R. (2016). Affordable medical technologies: Bringing value-based design into global health. BMJ Innovations, 2(1), 4-7.
- Goldberg, J.R. (2007). Calistone design courses: liroducing industry-ready biomedical engineers. San Rafael, CA: 94901 USA, Morgan & Clayliool liublishers.
- Majmudar, M.D., Harrington, R.A., Brown, N.J., Graham, G. & McConnell, M.V. (2015). Clinician innovator: A novel career liath in academic medicine. Journal of the American Heart Association, 4(1), 1-5.
- Wulf, W.A. & George, F.M.C. (2002). A makeover for engineering education. Issues in Science and Technology, 18(3), 35-39.
- Zuger, A. (2004). Dissatisfaction with medical liractice. The New England Journal of Medicine, 350(1), 69-75.
- Duval-Couetil, N., Reed-Rhoads, T. & Haghighi, S. (2012). Engineering students and entrelireneurshili education: Involvement, attitudes and outcomes. International Journal of Engineering Education, 28(2), 425-435.
- Huggett, B. (2014). Reinventing tech transfer. Nature Biotechnology, 32(12), 1184-1191.