Research Article: 2024 Vol: 27 Issue: 3S
A Critical Evaluation of USFDA Warning Letters Directed at Pharmaceutical Company
Gayatri H. Tiwaskar, Dadasaheb Balpande College of Pharmacy
Manasi Choudhari, Dadasaheb Balpande College of Pharmacy
Devashish Belankar, Dadasaheb Balpande College of Pharmacy
Ajay G. Pise, Dadasaheb Balpande College of Pharmacy
Citation Information: Tiwaskar G.H.,Choudhari M.,Belankar D., Pise A. G., (2024). A critical evaluation of USFDA warning letters directed at Pharmaceutical Company. Journal of Legal, Ethical and Regulatory Issues, 27(S3), 1-7.
Abstract
The pharmaceutical industry places significant importance on maintaining the quality of medications to ensure patient safety and effective treatment. However, the increasing number of warning letters issued by regulatory agencies such as the USFDA, TGA, and MHRA has raised concerns about the deteriorating quality of drugs. This article focuses on manufacturing quality, typical issues highlighted in USFDA483 observations, and the corrective and preventive measures implemented to address these issues. The Corrective and Preventive Action (CAPA) process plays a crucial role in identifying weaknesses, deviations, or incidents and taking immediate preventive action to prevent their recurrence. To improve quality culture, transparency with regulators during audits is essential. Analyzing warning letters issued to pharmaceutical companies is crucial in maintaining manufacturing quality across various drug products.
Keywords
Pharmaceutical industry, Quality, Patient safety, Warning letters, Regulatory agencies, USFDA, TGA, MHRA, Manufacturing quality, FDA483, Corrective action, Preventive action, CAPA, Quality culture, Transparency, Audits, Drug products.
Introduction
Quality can be defined as a product that meets established standards and specifications while adhering to cGMP regulations (U.S. Food and Drug Administration, 2005). According to the International Standards Organization (ISO), quality is the extent to which a set of attributes satisfies requirements (ISO, 2005).
Quality audits are structured and independent examinations that assess whether activities and results conform to planned arrangements. There are three main types of audits,
1. Internal
2. External, And
3. Regulatory Audits.
These serve as management tools for verifying objective evidence of processes, assessing implementation success, and judging effectiveness in achieving defined targets. Audits also provide evidence of problem areas (ICH, 2008).
A team consisting of audit inspectors and a multidisciplinary company team is required to conduct audits. The company team must include members from various departments such as production, quality control, warehousing, maintenance, administration, personnel, marketing, and sales (World Health Organization, 1997).
A regulatory audit report is a comprehensive evaluation to ensure that a project complies with regulatory guidelines and standards. According to the National Emergency Management Executive Academy (NEMEA) Compliance Centre, regulatory audits should be objective and independent to provide accuracy and assurance to the organization (Fundamentals & Vocabulary, 2005).
The Regulatory Audit Program (RAP) aims to examine programs and ensure that the procedures and compensation mechanisms comply with contractual and regulatory requirements (National Emergency Management Executive Academy, 2014).
Regulatory compliance refers to the adherence to rules and regulations. This concept has implications for the development of rules, regulations, and standards in all domains, including human services and economics (US Department of Defense, 2013).
FDA Form 483 is a document issued by the FDA at the conclusion of an inspection, informing the manufacturer of deviations from good manufacturing practices or other regulatory violations (Lane et al., 2006). The FDA Form 483 is followed by a timeline within which the manufacturer must take corrective actions to address the identified issues (US Food and Drug Administration, 2013).
The steps to be taken after receiving the FDA Form 483 include analyzing the findings, suggesting an appropriate timeline, assisting in charting a course of action, proposing corrective actions, and implementing them within the specified timeline. The company must also be available to answer any questions from the FDA during the correction process (Regulatory Compliance Associates, 2021).
Common Issues that Have Appeared in Fda 483
The most common violations of regulations occur specifically in different sections of 21 CFR part 210 and 211 which are as follows (Mauriello, 2017):
Section 501 (a) (2) (B) FD&C Act, 21 U.S.C. 351 (a) (2) (B)
Section 505 (a) FD&C Act, 21 U.S.C. 355(a)
Section 301 (d) of FD&C Act, 21 U.S.C. 331 (d)
Section 502 (c) and (x) FD&C act, 21 U.S.C 352 (c) and (x)
Section 301 (a) of FD&C act, 21 U.S.C 331 (a)
The Code of Federal Regulations (CFR) is a collection of rules and regulations established by federal agencies to implement federal laws. In the pharmaceutical industry, the most common violations of regulations occur in different sections of 21 CFR part 210 and 211. These regulations govern the manufacture, processing, packing, and holding of drug products (Code of Federal Regulations; FDA, 2018).
Section 501 (a) (2) (B) of the Federal Food, Drug, and Cosmetic (FD&C) Act, 21 U.S.C. 351 (a) (2) (B), states that a drug is considered adulterated if it is manufactured, processed, packed, or held in a facility that does not comply with current Good Manufacturing Practices (cGMPs). The cGMP regulations are designed to ensure that drug products are safe, effective, and of high quality.
Section 505 (a) of the FD&C Act, 21 U.S.C. 355(a), requires that a new drug be approved by the US Food and Drug Administration (FDA) before it can be marketed in the US. The FDA reviews the safety and efficacy data of the drug before making a decision on its approval.
Section 301 (d) of the FD&C Act, 21 U.S.C. 331 (d), prohibits the introduction or delivery for introduction into interstate commerce of any drug that is adulterated or misbranded.
Section 502 (c) and (x) of the FD&C Act, 21 U.S.C. 352 (c) and (x), require that the labeling of a drug product contain adequate directions for use and adequate warnings against use in certain conditions or by certain individuals.
Section 301 (a) of the FD&C Act, 21 U.S.C. 331 (a), prohibits the introduction or delivery for introduction into interstate commerce of any drug that is adulterated, misbranded, or unapproved.
It is important for pharmaceutical companies to comply with these regulations to ensure that their drug products are safe, effective, and of high quality. Failure to comply with these regulations can result in warning letters, recalls, and legal action by regulatory agencies.
CAPA
A system for analyzing, correcting, and preventing issues. It outlines procedures to solve the issue, it also analyzes the cause of the problem to prevent its recurrence. The result will be a thorough, well-documented investigation and solution that will meet the requirements of the law and form the basis for an effective continuity and improvement plan for any company (Baldwin, 2021).
Definition A/C To Q10
ICH Q10 describes one comprehensive model for an effective pharmaceutical quality system that is based on International Standards Organisation (ISO) quality concepts, includes applicable Good Manufacturing Practice (GMP) regulations, and complements ICH Q8 “Pharmaceutical Development” and ICH Q9 “Quality Risk Management”. The CAPA approach should lead to a product as well development process and an improved product as well cognitive process (Baldwin, 2021; Van-Trieste, 2011).
CAPA methodology is useful where corrective actions and preventive actions are incorporated into the iterative design and development process. CAPA can be used as an effective system for feedback, feedforward, and continual improvement. CAPA should be used, and the effectiveness of the actions should be evaluated (Abhishek, 2016).
Regulatory Aspects
Regulatory compliance is an important aspect of Corrective and Preventive Action (CAPA) procedures. In the United States, the FDA Code of Federal Regulations, Chapter 21, Part 820, specifically under Section 820.100, mandates the implementation of CAPA. In the European Union, the ISO 13485:2012 standard provides guidance on CAPA procedures under sections 8.5.2 for corrective measures and 8.5.3 for preventive action. Both the FDA and ISO 13485 require proper documentation of CAPA procedures (FDA, 2016).
The main objective of CAPA is to ensure that the procedures comply with the quality system regulation and are well-documented. It also involves analyzing data from relevant sources to identify actual product and quality issues requiring corrective action, as well as identifying potential product and quality-related problems requiring preventive action. Furthermore, CAPA aims to detect adverse trends and address them accordingly (Baldwin, 2021; Van-Trieste, 2011).
The Corrective and Preventive Action (CAPA) procedure is a critical component of a quality management system, and it is explicitly required by both the USFDA and ISO 13485. In the CAPA procedure, there are ten steps that must be followed to ensure compliance with regulatory requirements (FDA, 2016):
1. Verify that CAPA system procedure(s) that address the requirements of the quality system regulation have been defined and documented.
2. Determine if appropriate sources of product and quality problems have been identified. Confirm that data from these sources are analyzed to identify existing product and quality problems that may require corrective action.
3. Determine if sources of product and quality information that may show unfavorable trends have been identified. Confirm that data from these sources are analyzed to identify potential product and quality problems that may require preventive action.
4. Challenge the quality data information system. Verify that the data received by the CAPA system are complete, accurate and timely.
5. Verify that appropriate statistical methods are employed (where necessary) to detect recurring quality problems. Determine if results of analyses are compared across different data sources to identify and develop the extent of product and quality problems.
6. Determine if failure investigation procedures are followed. Determine if the degree to which a quality problem or nonconforming product is investigated is commensurate with the significance and risk of the nonconformity. Determine if failure investigations are conducted to determine root cause (where possible). Verify that there is control for preventing the distribution of the nonconforming product.
7. Determine if appropriate actions have been taken for significant product and quality problems identified from data sources.
8. Determine if corrective and preventive actions were effective and verified or validated prior to implementation. Confirm that corrective and preventive actions do not adversely affect the finished device.
9. Verify that corrective and preventive actions for product and quality problems were implemented and documented.
10. Determine if information regarding nonconforming product and quality problems and corrective and preventive actions has been properly disseminated, including dissemination for management review.
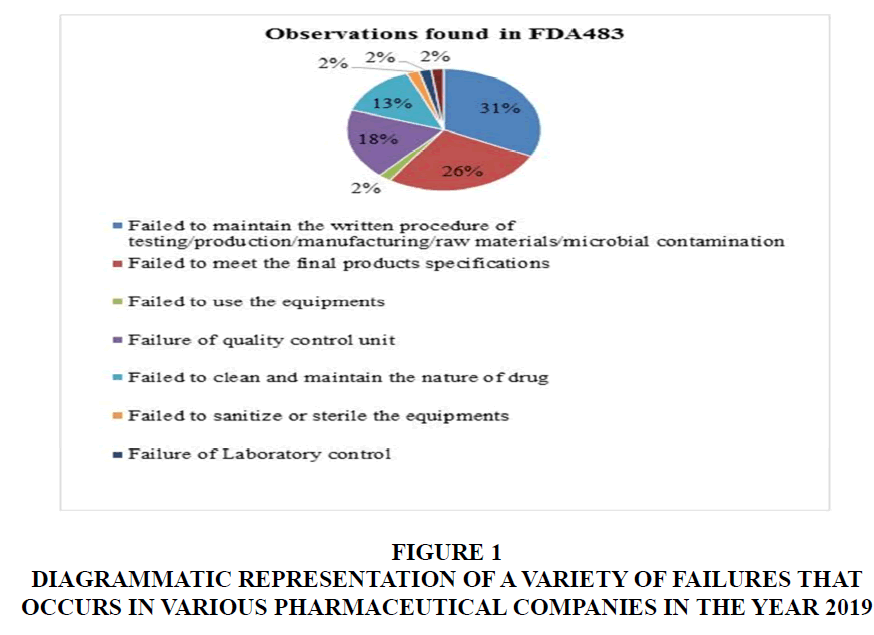
Following these steps can help ensure that firms comply with regulatory requirements and reduce the ratio of failure. Moreover, if pharmaceutical industries follow these guidelines strictly on a day-to-day basis in all aspects of their manufacturing, testing, and documentation, the ratio of the failure of firms can be easily decreased (Hussain, 2018). (Table 1) (Figure 1)
| Table 1 Different Issues Found in Some Companies | ||
| Sr. no. | Company Name | Issues |
| 1 | Lupin Limited MARCS-CMS 633703 — SEPTEMBER 27, 2022 | 1. Failure to establish adequate written procedures for cleaning equipment and its release for use in the manufacture of intermediates and API. 2. Failure to establish written procedures to monitor the progress and control the performance of processing steps may cause variability in the quality characteristics of your intermediates and API. 3. Failure to investigate all critical deviations. |
| 2 | Aurobindo Pharmaceutical Limited MARCS-CMS 618091 — JANUARY 12, 2022 | 1. Failure to evaluate the potential effect that changes may have on the quality of your intermediates and API. 2. Failure of your quality unit to ensure that critical deviations are investigated and resolved. |
| 3 | Panacea Biotec Limited MARCS-CMS 607837 SEPTEMBER 24, 2020 | 1. The firm failed to establish laboratory controls. 2. The firm did not establish an adequate system for monitoring environmental conditions in the aseptic processing area |
| 4 | Mayon's Pharmaceuticals Pvt Ltd MARCS-CMS 607388 SEPTEMBER 04, 2020 | 1. The firm failed to carry out at least one test to verify the identity of an individual component of a drug product. 2. The firm did not test each component for conformity with all appropriate specifications for purity, strength, and quality. 3. The firm failed to establish written procedures for production and process control. 4. The firm failed to Accomplish the cleanliness of the equipment and utensils and to prevent them from contamination or carry-over of a material that would affect and change the quality of the API beyond the official or other established specifications. |
| 5 | Windlass Healthcare Private Limited MARCS-CMS595494 MARCH 10, 2020 | 1. The firm failed to assure laboratory records included complete data derived from all tests. 2. The firm failed to establish an adequate quality control unit with the responsibility and authority to approve or reject all components. |
| 6 | Yibin Lihao Biotechnical Co., Ltd. MARCS-CMS 592503 FEBRUARY 13, 202 | 1. The firm failed to prepare and use the production and control records for each intermediate and API batch. 2. The firm does not establish, document, and implement an effective system for managing quality that involves the active participation of management and appropriate manufacturing personnel. |
| 7 | Sunstar Guangzhou Ltd. MARCS-CMS 592906 JANUARY 22, 2020 | The firm failed to perform, for each batch of a drug product, appropriate laboratory determination of satisfactory conformance to the final specifications for the drug product. |
Figure 1 Diagrammatic Representation of a Variety of Failures that Occurs in Various Pharmaceutical Companies in the Year 2019
Results and Discussion
The FDA483 reported that the pharmaceutical firms did not meet the criteria set by the regulatory authority regarding manufacturing quality. To avoid such issues, it is important for the firms to strictly adhere to the guidelines, rules, and regulations of the USFDA in all aspects of their operations, including manufacturing, testing, and documentation. Implementing the Corrective and Preventive Action (CAPA) methodology can also help firms address and prevent issues from recurring. By following these measures, firms can reduce their failure rates and ensure compliance with regulatory standards.
References
Abhishek, M. (2016). Quality Risk Management and CAPA. Journal of GXP Compliance, 20(4), 58-64.
Baldwin, J. (2021). Corrective and Preventive Action (CAPA) System. In Quality Management in the Imaging Sciences (pp. 141-151).
Indexed at, Google Scholar, Cross Ref
Code of Federal Regulations (CFR) - Title 21 - Food and Drugs.
FDA, (2018). Federal Food, Drug, and Cosmetic (FD&C) Act.
Fundamentals & Vocabulary, (2005). International Organization for Standardization. ISO 9000:2005.
Hussain, A. (2018). Importance of CAPA in pharmaceuticals. Journal of Pharmaceutical Sciences and Research, 10(1), 205-209.
ISO, (2005). ISO 9000:2005 - fundamentals and vocabulary. International Organization for Standardization; 2005.
Lane LM, Villarreal MC, Gomez JF, (2006). Regulatory compliance: developing a theoretical framework. J Bus Res. 2006;59(1):1-9.
Mauriello, S. (2017). FDA Form 483, What it is and What to do About it.
National Emergency Management Executive Academy (2014). Regulatory audit: a risk management tool. NEMEA Compliance Center.
Regulatory Compliance Associates (2021). FDA 483 response services. Regulatory Compliance Associates Inc.
U.S. Food and Drug Administration (2006). Quality Systems Approach to Pharmaceutical CGMP Regulations.
US Department of Defense, (2013). Regulatory audit program. Defense Contract Management Agency.
US Food and Drug Administration, (2013). Form FDA 483: inspectional observations. US Food and Drug Administration.
Van-Trieste, T. (2011). The CAPA Handbook: For those Who Have to Comply with FDA Regulations.
World Health Organization, (1997). Quality control of medicines: a guide to good manufacturing practice. World Health Organization.
Received: 02-Feb-2024, Manuscript No. JLERI-24-14582; Editor assigned: 03-Feb-2024, Pre QC No. JLERI-24-14582(PQ); Reviewed: 17-Feb-2024, QC No. JLERI-24-14582; Revised: 22-Feb-2024, Manuscript No. JLERI-24-14582(R); Published: 29-Feb-2024